Disorders
of consciousness
Our team assesses the recovery of neurological and physical disability, and of neuronal plasticity in patients with severe brain injuries. At the behavioral level, we assess the level of consciousness, pain perception and spasticity. At the cerebral level, multimodal functional neuroimaging is used to quantify neuronal preservation and plasticity. Our goal is to characterize the brain structure and residual cerebral function in patients who survive severe brain damage: patients in coma, as well as patients with unresponsive wakefulness syndrome (UWS), minimally conscious state (MCS) and locked-in syndrome (LIS).
The importance of this work is twofold. First, these patients represent a clinical challenge for diagnosis, prognosis, treatment and daily management. Second, these patients offer the opportunity to explore human consciousness, which is a significant conundrum neuroscience has yet to explain. Indeed, these patients present a complete, nearly graded range of states from unconsciousness (coma) to full awareness (locked-in syndrome).
Definition
Disorders of consciousness occur after a severe brain injury leading to a coma.
Diagnosis
Obtaining an accurate diagnosis has tremendous clinical and medico-ethical implications.
Prognosis
A reliable prognosis of patients with disorders of consciousness is a fundamental aspect of care.
Treatments
The field of treatment options for patients with disorders of consciousness suffers from a scarcity of evidence.
Definition
Disorders of consciousness occur after a severe brain injury leading to a coma. The etiologies vary and include traumatic brain injury, stroke or hypoxic/ischemic incidents.
During a coma, the patient is completely unresponsive to the environment, as witnessed at the bedside by the absence of eye-opening, even in response to painful stimulation (Plum and Posner 1972). As opposed to other states of transient unconsciousness (e.g., concussion, syncope), coma lasts for at least one hour. In contrast to popular belief, the coma period does not extend over several weeks. After this period the patient evolves to brain death or recovers at least some degree of arousal (wakefulness).
Brain death is defined as the irreversible loss of all functions of the brain, including the brainstem. Vital functions such as respiration, homeostatic regulation or cardiac function are also irreversibly impaired (Bernat 1998). The diagnosis is made within 6 to 24 hours post injury (Barclay 1981).
In 1972, Jennett and Plum introduced the term “Persistent Vegetative State” (PVS) to define patients who awoke from coma (i.e., opened their eyes) but who were unable to show intentional responses and solely demonstrated reflexes (e.g., limb withdrawal after painful stimulation) (Jennett and Plum 1972). While the term vegetative was initially chosen to describe the preservation of vegetative functions, it later appeared that the public’s attitude towards those patients was incorrectly associated as being vegetable-like. Responding to the need for a new name, the European Task Force on Disorders of Consciousness introduced the term « Unresponsive Wakefulness Syndrome ». This neutral term describes patients showing numerous clinical symptoms (hence the use of syndrome) of wakefulness without responsiveness (i.e., inability to show purposeful behaviors or command following while being awake, as shown by spontaneous or induced eye opening) (Laureys et al. 2010).
The diagnostic criteria for the Minimally Conscious State (MCS) were released in 2002 and based on an expert panel consensus, the Aspen Neurobehavioral Conference Workgroup (Giacino et al. 2002). The following behavioral features were described to diagnose MCS: following simple commands, ability to gesture or verbalize yes/no responses regardless of accuracy, intelligible verbalization, and showing purposeful behaviors following relevant stimuli that are distinguishable from reflexive responses (e.g., smiling or crying within the appropriate context, reaching for objects and/or using them, eye pursuit or fixation to moving stimuli).
Once the criteria were well established and applied, it appeared that the MCS clinical entity was heterogeneous. A wide range of behaviors was observed in these patients. Therefore, it was later subdivided into two clinical entities: the MCS plus (MCS+) and the MCS minus (MCS-). MCS+ is based on the presence of either command following, intelligible verbalization or intentional communication while MCS- includes the remaining MCS features, such as automatic movement, object manipulation or visual pursuit (Bruno et al. 2011).
When a patient regains the ability to show some complex behaviors such as functional communication (i.e., answer correctly six out of six situational, autobiographical or sensory-related questions) or functional use of objects (i.e., appropriately use two different objects) on two consecutive assessments, (s)he is considered to have emerged from the MCS (Giacino et al. 2002). These patients are usually still severely disabled and require full-time assistance. Emergence from the MCS (EMCS) is situated between the MCS and the fully conscious state, but the precise boundary is ill-defined.
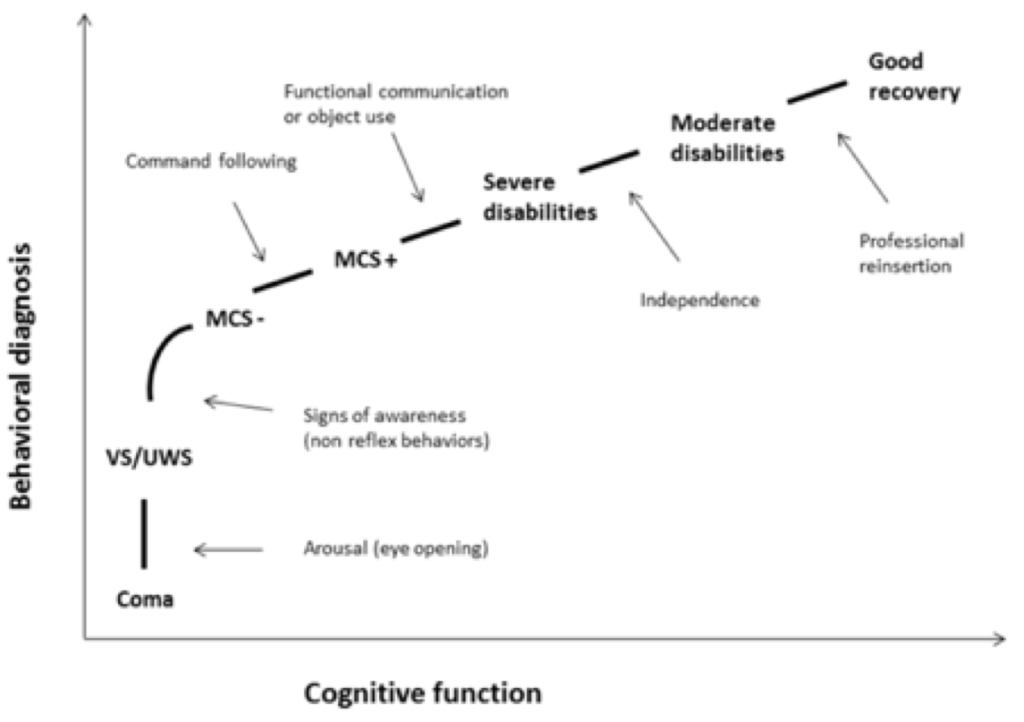
Path of recovery from coma, with gradually increasing levels of behavioral and cognitive functions. Taken from Chatelle and Laureys (2011), with authors’ permission. MCS=minimally conscious state; VS/UWS=vegetative state/unresponsive wakefulness syndrome
Références
- Barclay, W. R. (1981). Guidelines for the Determination of Death. JAMA J. Am. Med. Assoc. 246, 2194.
Bernat, J. L. (1998). A defense of the whole-brain concept of death. Hast. Cent Rep 28, 14–23. - Jennett, B., and Plum, F. (1972). Persistent vegetative state after brain damage. A syndrome in search of a name. Lancet 1, 734–737.
- Laureys, S., Celesia, G. G., Cohadon, F., Lavrijsen, J., Leon-Carrion, J., Sannita, W. G., et al. (2010). Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med 8, 68.
- Plum, F., and Posner, J. B. (1972). The diagnosis of stupor and coma. Contemp Neurol Ser 10, 1–286
- Bruno MA, Vanhaudenhuyse A, Thibaut A, Moonen G, Laureys S. J Neurol. (2011) From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: recent advances in our understanding of disorders of consciousness. Journal of Neurology; 258(7):1373-84.
- Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, Kelly JP, Rosenberg JH, Whyte J, Zafonte RD, Zasler ND. (2002) The minimally conscious state: definition and diagnostic criteria. Neurology. 58(3):349-53.
Diagnosis
Obtaining an accurate diagnosis has tremendous clinical and medico-ethical implications. From an ethical perspective, differential diagnoses of coma, UWS or MCS have consequences regarding end-of-life decisions, since prognosis differs for each condition. Several tools for the proper diagnosis of these patients are available. We can distinguish the diagnosis performed at the bedside, where a clinician will obtain behavioral information; and the complimentary diagnostic information provided by neuroimaging, where the clinician will obtain some objective data of cerebral integrity

At the bedside
Initially, the Glasgow Coma Scale (GCS) was developed to “assess the depth and duration of impaired consciousness and coma” (Teasdale and Jennett 1974). It consists of three subscales assessing motor responsiveness, verbal output and eye opening and can be performed in a couple of minutes. The total score ranges from 3 (deep coma) to 15 (normal consciousness). Later in the 1990s, a new generation of scales designed specifically for disorders of consciousness were developed, with varying levels of structure and standardization. This proliferation of new scales led Seel and colleagues to perform a systematic review (Seel et al. 2010) and identified 13 different assessment scales for patients with disorders of consciousness. The scale that best survived the ranking was the Coma Recovery Scale-Revised (CRSR) (Giacino, Kalmar, and Whyte 2004). Other scales such as the Sensory Modality Assessment and Rehabilitation Technique (SMART) (Gill-Thwaites and Munday 1999), the Wessex Head Injury Matrix (WHIM) (Shiel et al. 2000) or the Disorders of Consciousness Scale (DOCS) (Pape et al. 2005) were recommended with moderate reservations, while some were simply not recommended (e.g., Full Outline of UnResponsiveness; FOUR (Wijdicks et al. 2005), Glasgow-Liège Scale; GLS (Born 1988)).
The CRS-R is thus the recommended scale for the behavioral assessment of patients with disorders of consciousness. It consists of 23 items, hierarchically organized in six different subscales, assessing auditory, visual, motor, oro-motor functions, arousal and communication.
Behavioral fluctuation is a well-known phenomenon in this population (Candelieri et al. 2011; Piarulli et al. 2016). A key study regarding CRS-R evaluation in disorders of consciousness concerns the repetition of the assessments. Wannez and colleagues showed that the number of consecutive assessments had a significant impact on the clinical diagnosis: the risk of misdiagnosis after one assessment was 36% while the risk was reduced to 5% after 5 assessments. The authors therefore recommend performing at least five CRS-R assessments to obtain an accurate diagnosis (Wannez et al. 2017).
The time that should be allocated by clinicians to these evaluations is however rarely available in clinical conditions. We consequently developed a new short tool: the Simplified Evaluation of CONsciousness Disorders (SECONDs). This scale focuses on the 5 most frequently observed behaviors on the CRS-R (i.e., reproducible movement to command, visual pursuit, visual fixation, automatic motor response and localization to pain), which allows the detection of 99% of minimally conscious patients (Wannez et al., 2017b).
The SECONDs was validated in 57 patients in prolonged disorders of consciousness (Aubinet et al., 2021a), and the administration of the SECONDs was 2.5 times faster than the CRS-R. Our findings showed a substantial agreement between the SECONDs and the CRS-R diagnoses, as well as almost perfect agreements with regard to intra- and inter-rater reliability. More recently, we published an open access video paper providing the detailed guidelines and instructions for administrating the SECONDs in a standardized and reproducible manner (Sanz et al., 2021; https://www.jove.com/v/61968/seconds-administration-guidelines-fast-tool-to-assess-consciousness).
Overall, the SECONDs appears to be an appropriate tool to assess the level of consciousness in post-comatose patients, which could be easily implemented in clinical settings to decrease misdiagnosis in this challenging population. Please find below the available versions of the SECONDs in different languages; other translations will soon follow.
Our team further created new behavioral assessments to better identify patients’ co-morbidities. As regards language impairment, Aubinet et al. (2021b) elaborated the Brief Evaluation of Receptive Aphasia (BERA) tool, which examines the level of word and sentence comprehension (i.e., receptive phonological, semantic and morphosyntactic abilities with controlled psycholinguistic variables), based on the visual selection of a target-picture next to a specific distractor. This BERA tool was validated in a sample of conscious aphasic patients, and current studies focus on the development of an eye-tracked BERA version, as well as the validation of both tools in post-comatose patients. A substantial proportion of patients with disorders of consciousness also present severe dysphagia. The new SWallowing Assessment in Disorders Of Consciousness (SWADOC) was developed by Mélotte et al. (2021) to overcome the lack of a suitable tool that would allow clinicians to assess and measure swallowing-related components in these post-comatose patients. This assessment explores some of the oral and pharyngeal components of swallowing as well as a range of prerequisites and related components of swallowing, and includes both quantitative and qualitative items. An ongoing study is currently conducted to validate this tool in a large sample of post-comatose patients.
Find below the links to the BERA, CRS-R, FOUR, GOS-E, SECONDs and SWADOC:
Neuroimaging
Neuroimaging methods allow for structural, metabolic and electrophysiological investigation of the cerebral integrity and may complement the behavioral diagnosis. This is of particular interest in specific cases where executive functions are impaired and/or the deficit in motor abilities prevents the patient from showing any behavioral response while having at least partly preserved signs of consciousness. This situation, coined cognitive motor dissociation (CMD), covert consciousness or MCS* (Fernández-Espejo, Rossit, and Owen 2015; Gosseries, Zasler, and Laureys 2014; Laureys and Schiff 2012), can lead to a misdiagnosis rate of up to 32% (Stender et al. 2014). Therefore, the role of neuroimaging to detect responses invisible at the bedside is of paramount importance.
Magnetic resonance imaging (MRI) uses strong magnetic fields to form tridimensional representations of the brain’s structure and function. It is widely used in clinical settings to identify swelling, bleeding and other injuries in white and grey matter (Giacino et al. 2014; Kampfl et al. 1998). Conventional T1-weighted MRI is often qualitatively evaluated by neuro-radiologists (Morozova et al., 2018) or used to increase the spatial resolution of functional neuroimaging such as fMRI. Quantitative assessment of grey matter atrophy is rare, but proven useful for the assessment of DOC patients too. Subcortical atrophy in the thalamus and in the basal ganglia have been related to awareness and wakefulness respectively (Lutkenhoff et al., 2015). Atrophy in frontal cortical regions and the precuneus is more severe in UWS patients as compared to MCS patients (Guldenmund et al., 2016). Using regional grey and white matter volume, UWS and MCS patients could be automatically and successfully classified with similar classification accuracy as FDGPET, highlighting the clinical relevance of the assessment of cerebral structural integrity in DOC patients (Annen et al., 2018).
Functional MRI (fMRI) uses the blood oxygen level to monitor blood flow, as a proxy for neuronal activity (active neurons need more oxygen for metabolism) and thus measures cerebral processes with a high temporal resolution. In so-called active paradigms, cortical activation can be measured with fMRI following application of visual, auditory and/or somatosensory stimuli. In MCS patients, this is known to elicit activation of associative cortices, similar to what is observed in healthy controls (Di et al. 2008). In contrast, patients in UWS show activation only in primary sensory areas following the same stimuli (Di et al. 2007). fMRI is particularly interesting in the detection of CMD and could even, in some cases, be used to establish communication using « yes-no » active paradigms (Monti et al. 2010).
FDG-PET is another neuroimaging technique used to study the glucose uptake of cerebral areas, as a proxy for glucose metabolism. This can be used to quantify the global and regional brain metabolism, based on 18FDG uptake. Some brain areas are more crucial than others for recovery of consciousness (i.e., frontoparietal network, posterior parietal cortex and anterior cingulate cortices (Nakayama et al. 2006; Silva et al. 2010)). For diagnostics, FDG-PET seems to have better sensitivity and agreement with behavioral diagnosis compared to fMRI (Stender et al. 2014).

Recently, emphasis has been given on more affordable, user-friendly bedside neurophysiological tools such as electroencephalography (EEG). For example, measurements from high-density resting-state EEG (256 channels) are able to accurately discriminate between UWS, MCS and EMCS at the group level (Chennu et al. 2017) and also carry prognostic value. Patients with high connectivity (indicating strong connections between different cortical areas) in the delta frequency band (slow wave activity) tend to have negative outcomes at one year. In contrast, patients with positive outcomes have lower delta connectivity (Chennu et al. 2017).
Beyond resting-state, recording the EEG brain response after exposure to external perturbation such as transcranial magnetic stimulation (TMS) can contribute to characterization of a patient’s level of consciousness. Patients in UWS and in MCS present different responses to the magnetic trigger: a stereotypical slow wave that mostly remains local and short lasting for the former; a more widespread, differentiated and longer lasting wave for the latter (Rosanova et al. 2012). The perturbational complexity index (PCI) helps quantify these patterns by calculating the spatial and temporal brain response to the TMS perturbation. PCI successfully differentiates between conscious and unconscious states, with a clearcut difference at the individual level (Casali et al. 2013; Casarotto et al. 2016).
The range of complementary techniques for bedside behavioral diagnosis is thus wide. However, one must keep in mind the limits inherent to their use, including affordability, a high degree of expertise required for both signal acquisition and analysis, as well as the risks of false-negative/positive findings.
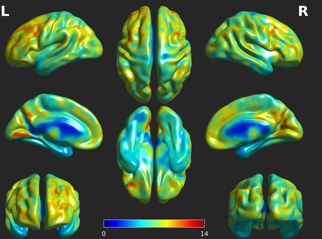
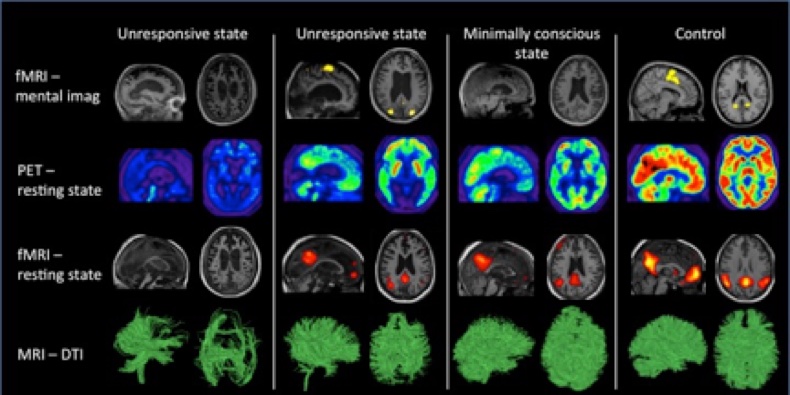
Illustrative summary of some of the neuroimaging techniques available for patients with disorders of consciousness across different diagnoses. Brain activity and structural integrity typically decreases with the level of consciousness. Adapted from Gosseries et al. (2014), with the permission of the authors.
Références
- Annen, J., Frasso, G., Crone, J.S., Heine, L., Di Perri, C., Martial, C., Cassol, H., Demertzi, A., Naccache, L., Laureys, S. (2018). Regional brain volumetry and brain function in severely brain‐injured patients. Ann Neurol., 83: 842-853.
- Born, J. D. (1988). The Glasgow-Liege Scale. Acta Neurochir. (Wien). 91, 1–11.
- Casali, A. G., Gosseries, O., Rosanova, M., Boly, M., Sarasso, S., Casali, K. R., et al. (2013). A theoretically based index of consciousness independent of sensory processing and behavior. Sci. Transl. Med. 5, 198ra105.
- Casarotto, S., Comanducci, A., Rosanova, M., Sarasso, S., Fecchio, M., Napolitani, M., et al. (2016). Stratification of unresponsive patients by an independently validated index of brain complexity. Ann. Neurol. 80, 718–729.
- Chennu, S., Annen, J., Wannez, S., Thibaut, A., Chatelle, C., Cassol, H., et al. (2017). Brain networks predict metabolism, diagnosis and prognosis at the bedside in disorders of consciousness. Brain 140, 2120–2132.
- Giacino, J. T., Kalmar, K., and Whyte, J. (2004). The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 85, 2020–2029.
- Candelieri, A., Cortese, M. D., Dolce, G., Riganello, F., and Sannita, W. G. (2011). Visual Pursuit: WithinDay Variability in the Severe Disorder of Consciousness. J. Neurotrauma 28, 2013–2017.
- Di, H., Boly, M., Weng, X., Ledoux, D., and Laureys, S. (2008). Neuroimaging activation studies in the vegetative state: predictors of recovery? Clin Med 8, 502–507.
- Di, H., Nie, Y., Hu, X., Tong, Y., Heine, L., Wannez, S., et al. (2014). Assessment of visual fixation in vegetative and minimally conscious states. BMC Neurol. 14, 147.
- Fernández-Espejo, D., Rossit, S., and Owen, A. M. (2015). A Thalamocortical Mechanism for the Absence of Overt Motor Behavior in Covertly Aware Patients. JAMA Neurol. 72, 1442.
- Giacino, J. T., Fins, J. J., Laureys, S., and Schiff, N. D. (2014). Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol 10, 99–114.
- Gosseries O, Zasler ND, Laureys S. Recent advances in disorders of consciousness: focus on the diagnosis. Brain Inj. 2014;28(9):1141-50.
- Gill-Thwaites, H., and Munday, R. (1999). The Sensory Modality Assessment and Rehabilitation Technique (SMART): a comprehensive and integrated assessment and treatment protocol for the vegetative state and minimally responsive patient. Neuropsychol. Rehabil. 9, 305–320.
- Guldenmund, P., Soddu, A., Baquero, K., Vanhaudenhuyse, A., Bruno, M.-A., Gosseries, O., Laureys, S., Gómez, F.(2016). Structural brain injury in patients with disorders of consciousness: A voxelbased morphometry study. Brain Injury, 30:3, 343-352,
- Kampfl, A., Schmutzhard, E., Franz, G., Pfausler, B., Haring, H.-P., Ulmer, H., et al. (1998). Prediction of recovery from post-traumatic vegetative state with cerebral magnetic-resonance imaging. Lancet 351, 1763–1767.
- Laureys, S., and Schiff, N. D. (2012). Coma and consciousness: Paradigms (re)framed by neuroimaging. Neuroimage 61, 478–491.
- Lutkenhoff, E.S., Chiang, J., Tshibanda, L., Kamau, E., Kirsch, M., Pickard, J.D., Laureys, S., Owen, A.M. and Monti, M.M. (2015). Thalamic and extrathalamic mechanisms of consciousness after severe brain injury. Ann Neurol., 78: 68-76.
- Monti, M. M., Vanhaudenhuyse, A., Coleman, M. R., Boly, M., Pickard, J. D., Tshibanda, L., et al. (2010).Willful modulation of brain activity in disorders of consciousness. N Engl J Med 362, 579–589.
- Morozova, S., Kremneva, E., Sergeev, D., Sinitsyn, D., Legostaeva, L., Iazeva, E., Krotenkova, M.,Ryabinkina, Y., Suponeva, N., Piradov, M. (2018). Conventional Structural Magnetic ResonanceImaging in Differentiating Chronic Disorders of Consciousness. Brain Sci, 2018, 8, 144.
- Nakayama, N., Okumura, A., Shinoda, J., Nakashima, T., and Iwama, T. (2006). Relationship between regional cerebral metabolism and consciousness disturbance in traumatic diffuse brain injury without large focal lesions: an FDG-PET study with statistical parametric mapping analysis. J Neurol Neurosurg Psychiatry 77, 856–862.
- Pape, T. L.-B., Heinemann, A. W., Kelly, J. P., Hurder, A. G., and Lundgren, S. (2005). A measure of neurobehavioral functioning after coma. Part I: Theory, reliability, and validity of the Disorders of Consciousness Scale. J. Rehabil. Res. Dev. 42, 1.
- Piarulli, A., Bergamasco, M., Thibaut, A., Cologan, V., Gosseries, O., and Laureys, S. (2016). EEG ultradian rhythmicity differences in disorders of consciousness during wakefulness. J. Neurol. 263, 1746–1760.
- Rosanova, M., Gosseries, O., Casarotto, S., Boly, M., Casali, A. G., Bruno, M. A., et al. (2012). Recovery of cortical effective connectivity and recovery of consciousness in vegetative patients. Brain 135, 1308–1320.
- Shiel, A., Horn, S. A., Wilson, B. A., Watson, M. J., Campbell, M. J., and McLellan, D. L. (2000). The Wessex Head Injury Matrix (WHIM) main scale: a preliminary report on a scale to assess and monitor patient recovery after severe head injury. Clin Rehabil 14, 408–416.
- Seel, R. T., Sherer, M., Whyte, J., Katz, D. I., Giacino, J. T., Rosenbaum, A. M., et al. (2010). Assessment Scales for Disorders of Consciousness: Evidence-Based Recommendations for Clinical Practice and Research. Arch. Phys. Med. Rehabil. 91, 1795–1813.
- Silva, S., Alacoque, X., Fourcade, O., Samii, K., Marque, P., Woods, R., et al. (2010). Wakefulness and loss of awareness: brain and brainstem interaction in the vegetative state. Neurology 74, 313–320.
- Stender, J., Gosseries, O., Bruno, M.-A., Charland-Verville, V., Vanhaudenhuyse, A., Demertzi, A., et al. (2014). Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study. Lancet 6736, 8–16.
- Teasdale, G., and Jennett, B. (1974). Assessment of Coma and Impaired Consciousness. Lancet 304, 81–84.
- Wijdicks, E. F., Bamlet, W. R., Maramattom, B. V, Manno, E. M., and McClelland, R. L. (2005). Validation of a new coma scale: The FOUR score. Ann Neurol 58, 585–593.
- Wannez, S., Heine, L., Thonnard, M., Gosseries, O., and Laureys, S. (2017b). The repetition of behavioral assessments in disorders of consciousness. Ann. Neurol. 81, 883–889
Prognosis
A reliable prognosis of patients with disorders of consciousness is a fundamental aspect of care. Surgical, medical and ethical decisions depend upon this information.
The outcome of patients in MCS is often better than the one of UWS patients (Faugeras et al, 2018). The cause of the brain injury also influences prognosis, as patients with a traumatic etiology such as a car accident are more likely to recover than patients with non-traumatic causes like cardiac arrest. Other prognostic factors are: a young age (Howell et al 2013), the CRS-R total score on admission (Estraneo et al 2013), an early time since onset (Whyte et al 2009), the presence of pupillary light reflexes (Fischer et al 2006), the absence of medical complications (Whyte et al 2013), and specialized early treatment (Seel et al 2013). Neuroimaging findings can also inform the prognosis. For instance, UWS patients who show preserved fMRI activation of associative cortices have higher chances to recover (Vogel et al 2013). The presence of long-latency event-related potential components in response to stimuli (Estraneo et al 2013) or preserved DMN connectivity (Norton et al 2012) are also indicative of a better recovery, while the bilateral absence of somatosensory evoked potentials in patients with cardiac arrest indicate a poor outcome (Robinson et al., 2003).
Références
- Estraneo A, Moretta P, Terme T, Trojano L. Predictors of recovery of responsiveness in prolonged anoxic vegetative state. Author reply. Neurology. 2013 Oct 1;81(14):1274-5.
- Fischer C, Luauté J, Némoz C, Morlet D, Kirkorian G, Mauguière F. Improved prediction of awakening or nonawakening from severe anoxic coma using tree-based classification analysis. Crit Care Med. 2006.
- Howell K, Grill E, Klein AM, Straube A, Bender A. Rehabilitation outcome of anoxic-ischaemic encephalopathy survivors with prolonged disorders of consciousness. Resuscitation. 2013 Oct;84(10):1409-15.
- Faugeras F, Rohaut B, Valente M, Sitt J, Demeret S, Bolgert F, Weiss N, Grinea A, Marois C, Quirins M, Demertzi A, Raimondo F, Galanaud D, Habert MO, Engemann D, Puybasset L, Naccache L. Survival and consciousness recovery are better in the minimally conscious state than in the vegetative state. Brain Inj. 2018;32(1):72-77.
- Norton L, Hutchison RM, Young GB, Lee DH, Sharpe MD, Mirsattari SM. Neurology. 2012 Jan 17;78(3):175-81Disruptions of functional connectivity in the default mode network of comatose patients.
- Robinson LR, Micklesen PJ, Tirschwell DL, Lew HL. Predictive value of somatosensory evoked potentials for awakening from coma. Crit Care Med. 2003 Mar;31(3):960-7.
- Seel RT, Douglas J, Dennison AC, Heaner S, Farris K, Rogers C. Specialized early treatment for persons with disorders of consciousness: program components and outcomes. Arch Phys Med Rehabil. 2013
- Vogel D, Markl A, Yu T, Kotchoubey B, Lang S, Müller F. Can mental imagery functional magnetic resonance imaging predict recovery in patients with disorders of consciousness? Arch Phys Med Rehabil. 2013
- Whyte J, Gosseries O, Chervoneva I, DiPasquale MC, Giacino J, Kalmar K, Katz DI, Novak P, Long D, Childs N, Mercer W, Maurer P, Eifert B.Predictors of short-term outcome in brain-injured patients with disorders of consciousness. Prog Brain Res. 2009;177:63-72.
- Whyte J, Nakase-Richardson R. Disorders of consciousness: outcomes, comorbidities, and care needs. Arch Phys Med Rehabil. 2013
Treatment
The field of treatment options for patients with disorders of consciousness suffers from a scarcity of evidence. This is due to the limited number of studies and because of the low class of evidence provided. To date, pharmacological and nonpharmacological treatments have been investigated in randomized controlled trials, open-label and case report studies. The aims of these studies are to improve patients’ level of consciousness and functional recovery, as well as to contribute to the understanding of the cerebral mechanisms of these interventions.
Pharmacological treatments
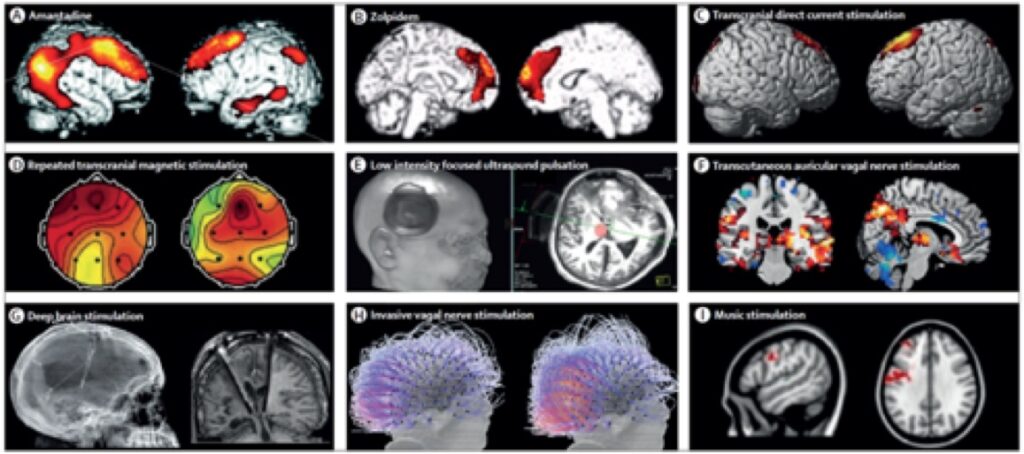
Only one pharmacological agent has been investigated in a large randomized controlled trial providing class II evidence: amantadine hydrochloride. Initially used as an antiviral and to treat Parkinson symptoms, it acts as a dopamine agonist and N-methyl-D-aspartate (NMDA) antagonist (Peeters et al. 2002). Its efficacy for disorders of consciousness has been shown in subacute patients who suffered from traumatic brain injury. Compared to placebo treated patients, the patients who received amantadine had a significantly faster recovery during the 4-week treatment period (Giacino et al. 2012).
Zolpidem is a GABA-agonist hypnotic agent that induces paradoxical responses in a few patients with disorders of consciousness. Dramatic, yet transient, improvements such as recovery of functional communication and reading abilities have been observed, but the rate of responders is extremely low. A first study reported only 5% of responders (Whyte et al. 2014). A higher proportion of 20% was described in another trial that included a large cohort of patients with disorders of consciousness. Improvements, without change of diagnosis, were observed, including recovery of response to command and object localization (Thonnard et al. 2014). Further studies are needed to understand why some patients respond to zolpidem and others do not.
Increasing interest has grown around apomorphine as a new treatment option. This non-selective dopamine agonist has a relatively short half-life (30 – 90 minutes (Kolls and Stacy 2006)) and is therefore recommended to be administered continuously through a subcutaneous pump (Katzenschlager et al. 2005). One case report and one prospective open-label trial reported fast and nearly complete cognitive and functional recovery in patients with disorders of consciousness (Fridman et al. 2009, 2010). Given the promising results, randomized controlled trials are forthcoming (Sanz et al. 2019).
Non-invasive brain stimulation
Transcranial direct current stimulation (tDCS) is certainly the most widely studied non-invasive neurostimulation technique. It uses weak electrical currents (1-2 mA) applied on the scalp to modify the excitability of targeted cortical areas. The direct current circulating between the anode and the cathode can modulate the brain activity and thereby improve the functions underpinned by the stimulated brain area (Stagg and Nitsche 2011). Dr Thibaut (co-director of the Coma Science Group) and colleagues conducted many clinical trials to test the effects of tDCS in patients with disorders of consciousness. About 30-50% of patients in MCS seem to positively respond to tDCS, but the effects remain limited (Thibaut et al. 2019). However, the individual response to tDCS is heterogeneous and montages targeting other areas than the prefrontal cortex should still be explored.
Another increasingly investigated non-invasive brain stimulation method is repeated TMS (rTMS). This technique uses an electromagnetic pulse to focally depolarize the neurons and induce firing. As with other brain stimulation methods, it allows for inhibition or activation of neuronal populations. A few randomized controlled trials targeting the motor cortex of patients with disorders of consciousness showed no clinical effects (Cincotta et al, 2015 ; He et al, 2018 ; Liu et al, 2018).
One case-report and two open-label studies, however, reported promising effects of rTMS on the dorsolateral prefrontal cortex (Louise-Bender Pape et al, 2009 ; Naro et al, 2015 ; Xia et al, 2017). Another recent open-label study reported positive effect of rTMS over the angular cortex (Legostaeva et al, 2019). Randomized controlled trials are currently being conducted to confirm these preliminary results.
Vagal nerve stimulation can also be administered in a non-invasive way through afferent branches located in the ear concha. A first single case report mentions using this technique in a chronic UWS patient who showed new signs of consciousness, after which the patient evolved to MCS within the next few weeks (Yu et al. 2017). Two other open-label studies showed promising results in a few patients (Noe et al. 2020, Hakon et al. 2020).
The Coma Science Group actively works on novel strategies using these non-invasive brain stimulations with optimized protocols to enhance patients’ recovery. We aim to better understand the mechanisms of action of the proposed interventions and induced-recovery, using behavioral tools and brain imaging techniques. An illustrative summary of available techniques is presented in the figure below.
Deep brain stimulation may be a popular intervention for patients with disorders of consciousness, following the renowned case report of Schiff and colleagues in 2007 (Schiff et al. 2007). This team surgically implanted electrodes in the intralaminar nuclei of the thalamus in a patient who was in MCS for six years after a traumatic brain injury. When the stimulator was on, important clinical improvements were observed, such as functional communication and successful oral feeding. When the stimulator was off, the clinical state of the patient decreased. Two other prospective open-label studies were conducted and reported moderate clinical improvements (Magrassi et al. 2016) and 29% of responders (i.e., emergence from MCS, response to command recovery) (Chudy et al. 2018). This appears encouraging but several challenges are reported as well, such as poor enrollment due to strict inclusion criteria, scalp infection and legal issues.
Vagal nerve stimulation is another neuromodulation technique that can be applied invasively with surgical implantation, but also non-invasively (Corazzol et al. 2017).
Palliative treatment
After severe brain injury leading to a disorder of consciousness, several primary and secondary complications may arise depending on the extent and the location of the cerebral lesions. When any part of the cortico-spinal neurons with long-range connections (upper motor neurons) is damaged, there is a risk to develop spasticity (Martens et al. 2017). Spasticity is defined as “a disordered sensori-motor control, resulting from an upper motor neurons lesion, presenting as intermittent or sustained involuntary activation of muscles” (Pandyan, Gregoric, and Barnes 2005). The clinical picture of patients suffering from spasticity is shown below.
Since this motor function impairment arises after a central lesion affecting the upper motor neurons, it is classically observed in patients with stroke, spinal cord injury, multiple sclerosis or traumatic brain injury. However, while its occurrence and pathophysiology are well-described in these populations, little is known for the cases involving more complex lesions leading to disorders of consciousness. In most of the cases, patients with disorders of consciousness are bedridden and suffer from paresis or paralysis, which favors the apparition of spasticity (by disuse and immobilization) and can provoke loss in range of motion, pain and bed sores (Martens et al. 2017).
A retrospective study collected all medical complications arising during rehabilitation in a large sample of individuals with disorders of consciousness following severe brain injury. Spasticity was, by far, the most common complication, affecting 70% of the participants. Next in line were autonomic nervous system dysregulation, epileptic seizure, hydrocephalus and intracranial infection (Nakase-Richardson et al. 2013).
Two other prospective studies documented the prevalence of spasticity in disorders of consciousness and found rates of 57% spastic patients (Ganesh et al. 2013) and 89% (Thibaut et al. 2014). Only a few studies investigated treatment options for spasticity in disorders of consciousness, using soft splints (Thibaut et al. 2015), acupuncture (Matsumoto-Miyazaki et al. 2016) and intrathecal baclofen (Francois et al. 2001; Shrestha et al. 2011). These techniques led to a clinically significant decrease in spasticity and thereby in the level of disability. They should therefore be considered in the palliative/comfort care arsenal for disorders of consciousness.
Pain perception represents another important medical and ethical issue, especially because patients with disorders of consciousness cannot communicate their experiences. Using brain imaging, MCS patients show a pain-matrix activation that is, although reduced, similar to what is seen in healthy volunteers while UWS patients do not show this higher-order, widespread brain activation. Unconscious patients would thus not feel pain like MCS patients and healthy volunteers do. The Nociception Coma Scale-Revised has been developed to assess pain in patients with disorders of consciousness. It includes the assessment of motor, verbal and facial behaviors (Chatelle et al. 2012). This scale has been shown to be able to selectively capture residual activity in pain matrix regions (e.g., anterior cingulate cortex) in these patients (Chatelle et al. 2014). At the bedside, if signs of discomfort are observed, pain medication should be given. Pain could however be experienced without demonstrating any behavioral sign of such discomfort. Therefore, pain prophylaxis and treatment should be proposed for all patients suffering from disorders of consciousness.
(Adapted from Géraldine Martens PhD thesis 2020)
Références
- Chatelle, C., Majerus, S., Whyte, J., Laureys, S., and Schnakers, C. (2012). A sensitive scale to assess nociceptive pain in patients with disorders of consciousness. J Neurol Neurosurg Psychiatry 83, 1233–1237.
- Chatelle C, Thibaut A, Bruno MA, Boly M, Bernard C, Hustinx R, Schnakers C, Laureys S. (2014). Nociception coma scale-revised scores correlate with metabolism in the anterior cingulate cortex. Neurorehabil Neural Repair.
- Chudy, D., Deletis, V., Almahariq, F., Marčinković, P., Škrlin, J., and Paradžik, V. (2018). Deep brain stimulation for the early treatment of the minimally conscious state and vegetative state: experience in 14 patients. J. Neurosurg.
- Cincotta, M., Giovannelli, F., Chiaramonti, R., Bianco, G., Godone, M., Battista, D., et al. (2015). Noeffects of 20 Hz-rTMS of the primary motor cortex in vegetative state: A randomised, shamcontrolled study. Cortex. 71, 368–376.
- Corazzol, M., Lio, G., Lefevre, A., Deiana, G., Tell, L., André-Obadia, N., et al. (2017). Restoringconsciousness with vagus nerve stimulation. Curr. Biol.
- Francois, B., Vacher, P., Roustan, J., Salle, J. Y., Vidal, J., Moreau, J. J., et al. (2001). Intrathecal baclofenafter traumatic brain injury: Early treatment using a new technique to prevent spasticity. J.Trauma – Inj. Infect. Crit. Care 50, 158–161.
- Fridman, E. A., Calvar, J., Bonetto, M., Gamzu, E., Krimchansky, B. Z., Meli, F., et al. (2009). Fastawakening from minimally conscious state with apomorphine. Brain Inj 23, 172–177.
- Fridman, E. A., Krimchansky, B. Z., Bonetto, M., Galperin, T., Gamzu, E. R., Leiguarda, R. C., et al. (2010).Continuous subcutaneous apomorphine for severe disorders of consciousness after traumaticbrain injury. Brain Inj. 24, 636–641.
- Giacino, J. T., Whyte, J., Bagiella, E., Kalmar, K., Childs, N., Khademi, A., et al. (2012). Placebo-controlledtrial of amantadine for severe traumatic brain injury. N. Engl. J. Med. 366, 819–826.
- He F, Wu M, Meng F, Hu Y, Gao J, Chen Z, Bao W, Liu K, Luo B, Pan G. (2018) Effects of 20 Hz RepetitiveTranscranial Magnetic Stimulation on Disorders of Consciousness: A Resting-StateElectroencephalography Study. Neural Plast.
- Katzenschlager, R., Hughes, A., Evans, A., Manson, A. J., Hoffman, M., Swinn, L., et al. (2005).Continuous subcutaneous apomorphine therapy improves dyskinesias in Parkinson’s disease:A prospective study using single‐dose challenges. Mov. Disord. 20, 151–157.
- Kolls, B. J., and Stacy, M. (2006). Apomorphine: A Rapid Rescue Agent for the Management of Motor Fluctuations in Advanced Parkinson Disease. Clin. Neuropharmacol. 29, 292–301.
- Hakon J, Moghiseh M, Poulsen I, Øland CML, Hansen CP, Sabers A. (2020) Transcutaneous Vagus Nerve Stimulation in Patients With Severe Traumatic Brain Injury: A Feasibility Trial. Neuromodulation.
- Ganesh, S., Guernon, A., Chalcraft, L., Harton, B., Smith, B., and Louise-Bender Pape, T. (2013). Medical comorbidities in disorders of consciousness patients and their association with functional outcomes. Arch Phys Med Rehabil 94, 1899–1907.
- Legostaeva L, Poydasheva A, Iazeva E, Sinitsyn D, Sergeev D, Bakulin I, Lagoda D, Kremneva E, Morozova S, Ryabinkina Y, Suponeva N, Piradov M. (2019) Stimulation of the Angular Gyrus Improves the Level of Consciousness. Brain Sci.
- Liu, P., Gao, J., Pan, S., Meng, F., Pan, G., Li, J., et al. (2016b). Effects of High-Frequency Repetitive Transcranial Magnetic Stimulation on Cerebral Hemodynamics in Patients with Disorders of Consciousness: A Sham-Controlled Study. Eur. Neurol. 76, 1–7.
- Martens, G., Laureys, S., and Thibaut, A. (2017). Spasticity management in disorders of consciousness. Brain Sci. 7, 162.
- Matsumoto-Miyazaki, J., Asano, Y., Ikegame, Y., Kawasaki, T., Nomura, Y., and Shinoda, J. (2016). Acupuncture Reduces Excitability of Spinal Motor Neurons in Patients with Spastic Muscle Overactivity and Chronic Disorder of Consciousness Following Traumatic Brain Injury. J. Altern. Complement. Med. 22, 895–902.
- Nakase-Richardson, R., McNamee, S., Howe, L. L., Massengale, J., Peterson, M., Barnett, S. D., et al. (2013). Descriptive characteristics and rehabilitation outcomes in active duty military personnel and veterans with disorders of consciousness with combat- and noncombat-related brain injury. Arch. Phys. Med. Rehabil. 94, 1861–1869. 15
- Naro A, Russo M, Leo A, Bramanti P, Quartarone A, Calabrò RS. (2015) A Single Session of Repetitive Transcranial Magnetic Stimulation Over the Dorsolateral Prefrontal Cortex in Patients With Unresponsive Wakefulness Syndrome: Preliminary Results. Neurorehabil Neural Repair.
- Noé E, Ferri J, Colomer C, Moliner B, O’Valle M, Ugart P, Rodriguez C, Llorens R. (2020) Feasibility, safety and efficacy of transauricular vagus nerve stimulation in a cohort of patients with disorders of consciousness. Brain Stimul.
- Pandyan, A. D., Gregoric, M., and Barnes, M. P. (2005). Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil 27, 2–6.
- Pape T, Rosenow J, Lewis G, Ahmed G, Walker M, Guernon A, Roth H, Patil V. (2009)Repetitive transcranial magnetic stimulation-associated neurobehavioral gains during coma recovery. Brain Stimul.
- Peeters, M., Page, G., Maloteaux, J.-M., and Hermans, E. (2002). Hypersensitivity of dopamine transmission in the rat striatum after treatment with the NMDA receptor antagonist amantadine. Brain Res. 949, 32–41.
- Sanz, L., Lejeune, N., Thibaut, A., Blandiaux, S., Stender, J., Farber, N., et al. (2018). Treating severely brain-injured patients with apomorphine: study protocol for a double blind randomized placebo-controlled trial using behavioral and neuroimaging assessments. Front. Neurosci. 12.
- Shrestha, P., Malla, H., Pant, B., and Taira, T. (2011). Intrathecal baclofen therapy in severe head injury, first time in Nepal, a technique suitable for underdeveloped countries. Asian J. Neurosurg. 6, 49–51.
- Stagg, C. J., and Nitsche, M. A. (2011). Physiological basis of transcranial direct current stimulation. Neuroscientist 17, 37–53.
- Thibaut, A., Bruno, M.-A., Ledoux, D., Demertzi, A., and Laureys, S. (2014). tDCS in patients with disorders of consciousness Sham-controlled randomized double-blind study. Neurology 82, 1112–1118.
- Thibaut, A., Chatelle, C., Wannez, S., Deltombe, T., Stender, J., Schnakers, C., et al. (2015a). Spasticity in disorders of consciousness: A behavioral study. Eur J Phys Rehabil Med 51, 289–397.
- Thibaut, A., Deltombe, T., Wannez, S., Gosseries, O., Ziegler, E., Dieni, C., et al. (2015b). Impact of soft splints on upper limb spasticity in chronic patients with disorders of consciousness: A randomized, single-blind, controlled trial. Brain Inj. 29, 830–836.
- Thibaut, A., Schiff, N., Giacino, J., Laureys, S., and Gosseries, O. (2019b). Therapeutic interventions in patients with prolonged disorders of consciousness. Lancet Neurol. 18, 600–614.
- Thonnard, M., Gosseries, O., Demertzi, A., Lugo, Z., Vanhaudenhuyse, A., Bruno, M.-A. A., et al. (2014). Effect of zolpidem in chronic disorders of consciousness: A prospective open-label study. Funct Neurol 28, 259–264.
- Whyte, J., Rajan, R., Rosenbaum, A., Katz, D., Kalmar, K., Seel, R., et al. (2014). Zolpidem and restoration of consciousness. Am J Phys Med Rehabil 93, 101–113.